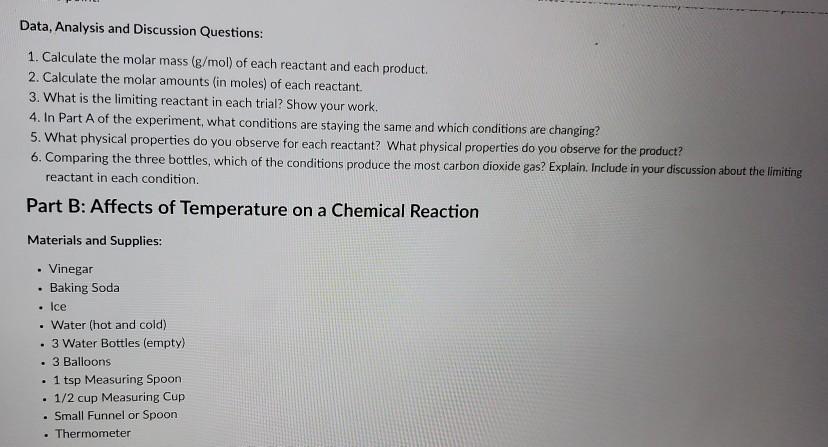
Baking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflateThis is a easy science experiments that shows what happens when a base and an acid meet Watch in amazement as the balloons inflate themselves Just 2 ingredWhat is the conclusion of mixing baking soda and vinegar?
Www State Nj Us Education 21cclc Casp Lsc Unit1 All Pdf
Baking soda vinegar balloon experiment explanation
Baking soda vinegar balloon experiment explanation-This experiment did not have any problems or issuesIts very interesting and fun Why does vinegar and baking soda inflateThe experiment and result of it supported our hypothesis that the bubbles would float on top of the mixture of the baking soda and vinegar It did this because when
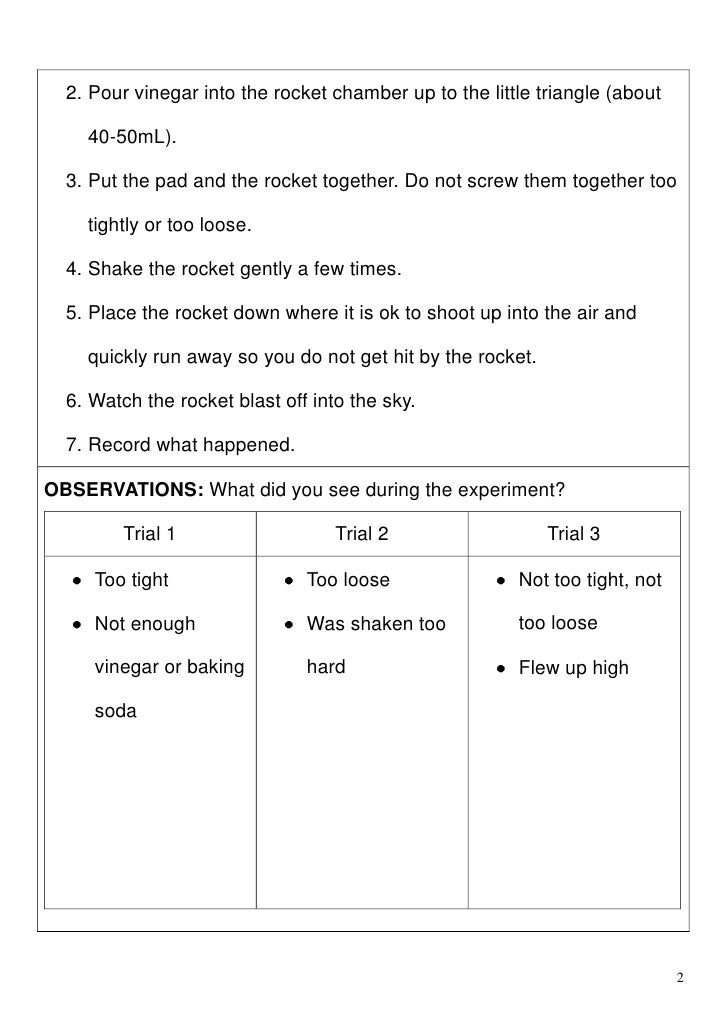



Baking Soda And Vinegar Experiment Report
What is the conclusion on inflate a balloon with vinegar and baking soda?The science, behind this balloon baking soda experiment, is the chemical reaction between the base {baking soda} and the acid {vinegar} When the two ingredients mix together the balloon baking soda experiment gets it's lift!When the vinegar and baking soda combine there is a reaction between an acid and a base Vinegar is the acid and baking soda is the base This reaction between the two causes a gas called carbon dioxide to bubble and foam This gas having nowhere else to go, expands the balloon making the selfinflating balloon happen
The baking soda should fall out of the balloon, through the neck of the bottle, and into the vinegar at the bottom Here, the two chemicals will fizz and react, turning into other chemicals One of these is carbon dioxide, a gas, which will rise up and inflate the balloonThat lift is the gas produced from the two ingredients is carbon dioxide or CO2The balloon should inflate because adding the lemon juice/vinegar to the bicarbonate of soda creates a chemical reaction, when the two combine they create the gas carbon dioxide Always encourage your kids to ask questions and wonder about what will happen if the vinegar has special stuff in it that makes that baking soda explode First, fill the water bottle about 1/3 of the
2 Mix the compounds in the beaker and wait for the reaction to finish 3 Record the mass of the beaker and its contents 4 Determine the remaining mass of the chemicals in the beaker 5 Measure the mass of a new beaker, a plastic weight boat, 10mL vinegar, and 10g baking soda 6Pour the vinegar into the bottle Carefully fit the balloon over the bottle opening (be careful not to drop the baking soda into the vinegar yet) Once the balloon is fitted snugly on the nozzle, hold up the balloon and allow the baking soda to fall into the vinegar Observe the chemical reaction and effect on the balloonVinegar, Baking Soda, and a Balloon!



Http Kendall Ipsd Org Uploads Kendall Sciencefairguidelines Pdf




How To Make A Heavy Balloon Introduction We
Measure 15 tsp or 875 grams of baking soda Insert a funnel into the opening of a balloon and add the baking soda to the balloon through the funnel See image to the right Measure 10 tbsp or approximately 150ml of vinegar Add the vinegar to the empty bottle If you wish to add food coloring to the vinegar, add 35 dropsVinegar Experiment School of Fun Series Learning Worksheets This printable is part of HP's School of Fun Series Tap here to see moreIn conclusion, the evidence shows that my hypothesis is correct Baking soda and vinegar do create CO2 Different amounts of each of the ingredients create more or less amounts of CO2 Another addition to this project would be to




Kim S Self Inflating Balloon Experiment Kesta Fleming




Baking Soda And Vinegar S Reaction Perkins Elearning
Science Experiments for Kids – Inflating a Balloon with Baking Soda and Vinegar Step 1 Add 2 or 3 teaspoons of baking soda to the unfilled balloon Step 2 Thoroughly rinse and dry the funnel Step 3 Pour 2 or 3 teaspoons of vinegar to the water bottle using the funnel Step 4 Attach the balloon to the top of the water bottle, beingIn conclusion, the average fastest time was for the 1/2 cup of baking soda Using the funnel, put the baking soda into the balloon 2 Last week we tested these two chemicals with red cabbage indicator, and found that a solution of bicarbonate of soda was alkaline, but vinegarBaking soda is bicarbonate (NaHCO3) and vinegar is acetic acid (HCH3COO) One of the products this reaction creates is carbon dioxide, which makes the bubbles When the baking soda meets the vinegar, there is a chemical reaction as carbon dioxide gas is created and fills the balloon causing it to inflate Carbon dioxide is an important gas in




Baking Soda And Vinegar Experiment Report




Balloon Baking Soda Vinegar Science Experiment For Kids
Directions Pour ARM &HAMMER Baking Soda into your balloon, filling the balloon halfway Use the funnel to pour vinegar into the water bottle, filling about ⅓ of the bottle Cover the top of the bottle with the bottom of the balloon When ready, lift the balloon and let the ARM &HAMMER Baking Soda fall into the vinegar



3



Www Northmasonschools Org Userfiles 97 Classes 2170 Bakingsodaandvinegarballoonexperiment Pdf Id 9642
Pour the baking soda into the balloon using a funnel Measure 45 ml of vinegar and pour it into a water bottle Put the mouth of the balloon on the wine spout to keep the baking soda in the balloon (The balloon will be flopped to one side) Lift the balloon up and pour the baking soda into the bottle of vinegar Repeat for each type of vinegarThe gas produced from the two ingredients is carbon dioxide or CO2The baking soda and the vinegar create an acidbase reaction and the two chemicals work together to create a gas, (carbon dioxide) Gasses need a lot of room to spread out and the carbon dioxide starts to fill the bottle, and then moves into the balloon to inflate it What might have caused the reaction to stop?




Law Of Conservation Of Mass Lab Purpose




What Happens When You Mix Vinegar And Baking Soda Wonderopolis
Baking SodaVinegarBalloons= A recipe for fun!Engage your students while teaching them about the scientific method with this fun Balloon Lab Students will have the opportunity to work in small groups, review lab safety, and learn how to run an experimentThe experiment and result of it supported our hypothesis that the bubbles would float on top of the mixture of the baking soda and vinegar It did this because when we combined the baking soda and vinegar it had a chemical reaction that produces carbon dioxide gasWhen vinegar and baking soda are mixed together they create what is called a chemical reaction A gas is formed (carbon dioxide) which takes up more space than the vinegar and baking soda That is why the balloon inflates Other points to share if doing this experiment with older children The baking soda is a base while the vinegar is an acid




How To Inflate A Balloon Using Baking Soda And Vinegar




Baking Soda Vinegar Balloon Worksheets Teaching Resources Tpt
Vinegar Balloon Experiment Stretch out the balloon a little bit Spread the mouth of the balloon open with your fingers (or utilize a funnel, if you have one on hand) and pour a teaspoon (or two) of baking soda into the balloon Fill the empty plastic bottle about 1/4 to 1/2full with vinegar While making sure to keep the bakingIn the balloon vinegar baking soda experiment, you will learn about the reactions between vinegar and baking soda You will also learn about the gas produced when combining vinegar and baking soda Balloon Blowing Up Magically Materials required BalloonThis Giant Balloon Baking Soda and Vinegar Experiment is a fun science project for kids for them to learn about acid base reactions with an entertaining twist I'm sure you've seen the classic balloon baking soda and vinegar experiment where you mix vinegar and baking soda together in a bottle




I Just Completed An Online Biochem Lab And I Am Chegg Com




Baking Soda Vinegar Experiment By Pat Acorda
What to do use a funnel to pour vinegar into your bottle (need to fill about 1/3 of the bottle) Pour baking soda into your balloon (fill the balloon approx 1/2 way) Cover the top of the bottle and lift your balloon and let the baking soda fall into the vinegarThe science, behind this balloon baking soda experiment, is the chemical reaction between the base – baking soda – and the acid – vinegar When the two ingredients mix together the balloon baking soda experiment gets its lift!17 $300 Zip Fun Science Experiments // Baking Soda and Vinegar Balloon ExperimentIncludes BOTH US size and Australian sized files/spelling!This fun, simple science experiment is an excellent hands on cost effective way to show children the three different states of matter and chemical changes in action!This




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities



Www Uen Org Lessonplan Print Pdf
This science experiment of balloon, vinegar and baking soda also explains that the carbondioxide is evolved when the mixture comes into contact Follow the steps for experiment of Balloon, Vinegar and Baking Soda 1) Take a clean plastic or glass bottle and see to that you are able to hold the balloon cap on to it firmlyThe baking soda will quickly react with the vinegar in the flask, creating carbon dioxide gas as one of its products, causing the balloon to quickly inflate After the reaction is complete the balloon will remain inflate What is the conclusion of mixing baking soda and vinegar?How to Blow Up a Ballon with Baking Soda &
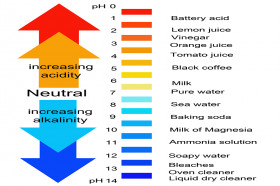



What Happens When You Mix Vinegar And Baking Soda Wonderopolis




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities
Use the small funnel to put 4 ounces of white vinegar in each water bottle Place Bottle 2 in the bowl of ice After three minutes place the balloon on top of the bottles, being careful not to spill the baking soda into the bottles yetSelfInflating Balloons From what I have seen,my hypothesis is rightThe balloon expanded and got biggerI learned that the vinegar and baking soda turns into carbonic acidThe carbonic acid is unstable and decomposes to become carbon dioxide and waterSince the carbon dioxide is less dense,it stretches the balloonThis is a very fun and easy science experiment for kids!!!Hey Everyone and welcome back to the labIn thi




Baking Soda And Vinegar Balloons
.jpg)



How To Get The Best Baking Soda And Vinegar Reaction
The conclusion to this experiment is that mixing an acid and a base will blow up an object What's happening here is that the vinegar which is an acid is making a chemical reaction with the baking soda, a base After the two substances mix you get a carbonic acid forcing the balloon to blow up What happens when you mix an acid and a baseClaim As the amount of vinegar is increased, the time it takes for the reaction between baking soda and vinegar reduces Evidence When one fluid ounce of vinegar was used, it took 544 seconds for one tablespoon of baking soda to cease its reaction with the vinegarTowards the end of the reaction, I could see leftover baking soda that had not taken part in the reactionLift and straighten the balloon so that the baking soda inside falls into the bottle of vinegar Wait until the balloon stops expanding Pinch the neck of the balloon closed and tie it off Record the mass of the tied balloon Repeat steps 26 twice Repeat steps 26 three times, substituting the 5ml of baking soda for 10ml of baking soda




Lesson 3 Balloon Experiment




What S The Difference Between Baking Soda And Baking Powder American Chemical Society
In conclusion I learned that water will ruin any reaction between a base and an acid (baking soda is a base and vinegar is an acid), and when you add heat to an acid and base explosion it will make the reaction bigger Hint If you are ever making a vinegar and baking soda bom, get something to heat it up firstChemical reaction science experiments using baking soda and vinegar are a lot of fun and are great learning opportunities In this quick and easy experiment, we are going to use an endothermic chemical reaction and the resulting carbon dioxide caused by mixing baking soda and vinegar to inflate a balloon Materials Empty plastic or glass bottle Balloon 1 cupRead MoreThe Baking Soda Balloon BlowUp Experiment Fill a water bottle onethird full of vinegar Put a funnel in the neck of a balloon, and hold onto the balloon neck and funnel Have your child pours in enough baking soda to fill the balloon halfway Slide the funnel out of the balloon and have your child hold the portion of the balloon with the



2




Vinegar And Baking Soda Balloon Experiment Happy Brown House
These baking soda vinegar experiment are fun for toddlers, preschoolers, kindergartners, grade 1, and grade 2 students as they don't require any fancy supplies and don't require toxic chemicals Instead these baking powder experiments make science fun for kids with creative uses of baking soda and vinegar From baking soda vinegar balloonFor this experiment you need a few things 1 Baking Soda 2 Vinegar 3 oz pop bottle 4 Spoon 5 Funnel 6 Balloon 7 Clothes PinUse a funnel to add 1/3 cup baking soda to the inside of a balloon Fill a plastic bottle with approximately 1 cup vinegar Attach the balloon to the mouth of the plastic bottle, then lift



Baking Soda Vinegar Balloon Experiment Capturing Parenthood




Law Of Conservation Of Mass Baking Soda And Vinegar Experiment With Balloon Simple Chemistry Classroom Middle School Science Teacher Middle School Science
Summary Vinegar is placed in a soda bottle and a baking soda filled balloon is attachedThe mixing of the baking soda and vinegar results in the balloon expanding Estimated Time 15 – minutes Materials Needed 1 5 L (169 oz) clean, empty, plastic soda bottleStep 2 Using paper funnel, pour baking soda into the balloon Try not to spill 14 Step 3 With out spilling the baking soda, put balloon on the bottle's mouth piece 15 Step 4 When ready, lift your balloon and let the baking soda fall into the vinegar 16




Balloon Blow Up Science Experiment




Baking Soda Vinegar Balloon Experiment Conclusion




Science Project How To Inflate Up A Balloon With Liquid Ppt Video Online Download



Baking Soda And Vinegar
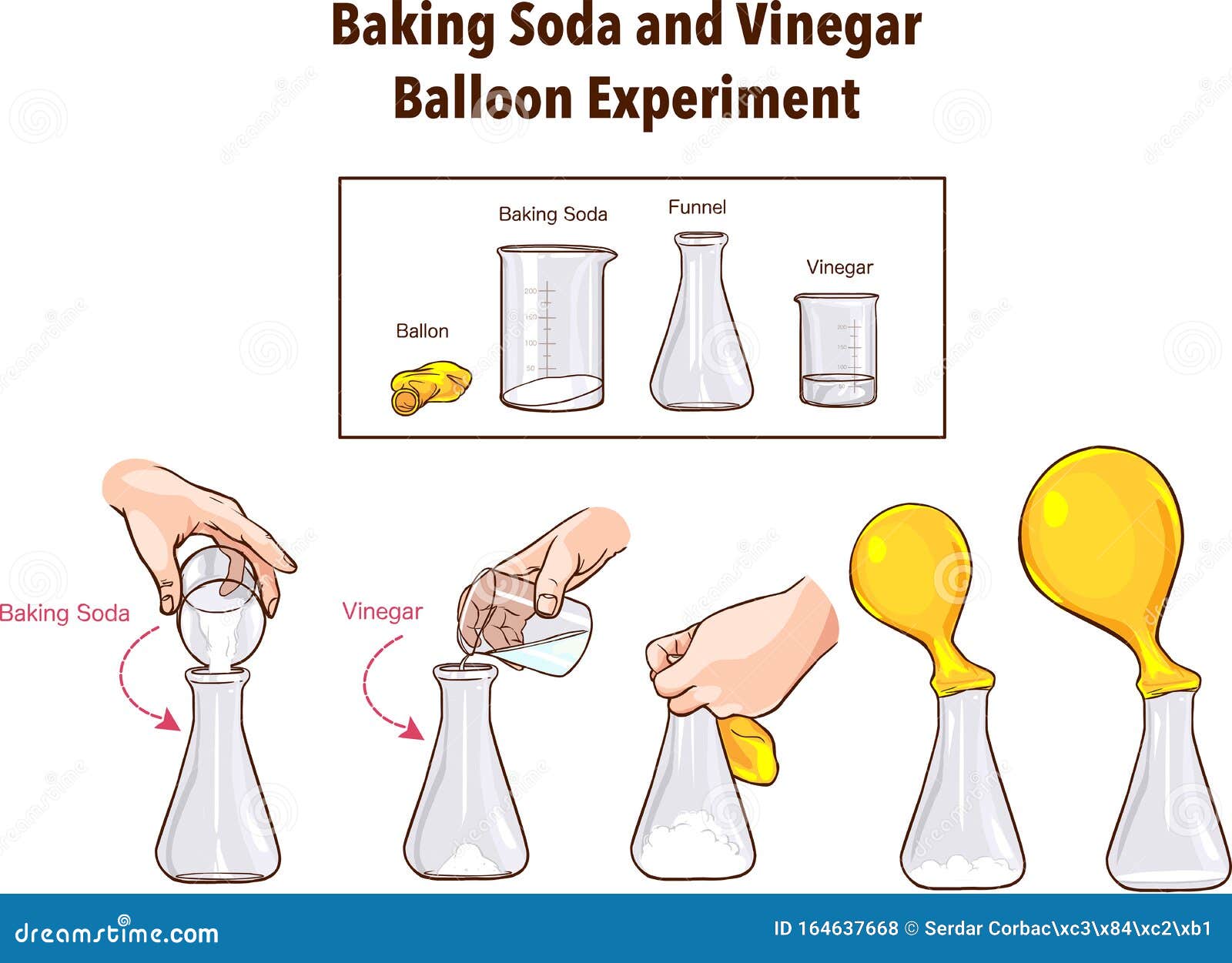



Baking Soda And Vinegar Balloon Experiment Science Stock Vector Illustration Of Background School



Www Northmasonschools Org Userfiles 97 Classes 2170 Bakingsodaandvinegarballoonexperiment Pdf Id 9642




Chemical Or Physical Change Perkins Elearning




Baking Soda And Vinegar Balloons




Baking Soda And Vinegar Lab Report Docx Baking Soda And Vinegar Lab Report Research Question What Is The Effect That Baking Soda Has On The Volume Of Course Hero



1




Please Help With The 1 Data Analysis And Chegg Com




Baking Soda And Vinegar Experiment Report




Pdf Diet Coke And Mentos What Is Really Behind This Physical Reaction




Science The Scientific Method Lesson 3 The Scientific Method Balloon Experiment Ppt Download




Use Vinegar And Baking Soda To Blow Up A Balloon Discovery Express




Minilab Limiting Reagents Versus Excess Reagents Ppt Video Online Download




Self Inflating Balloon By Adoomi Zh



Science Lab Science Isn
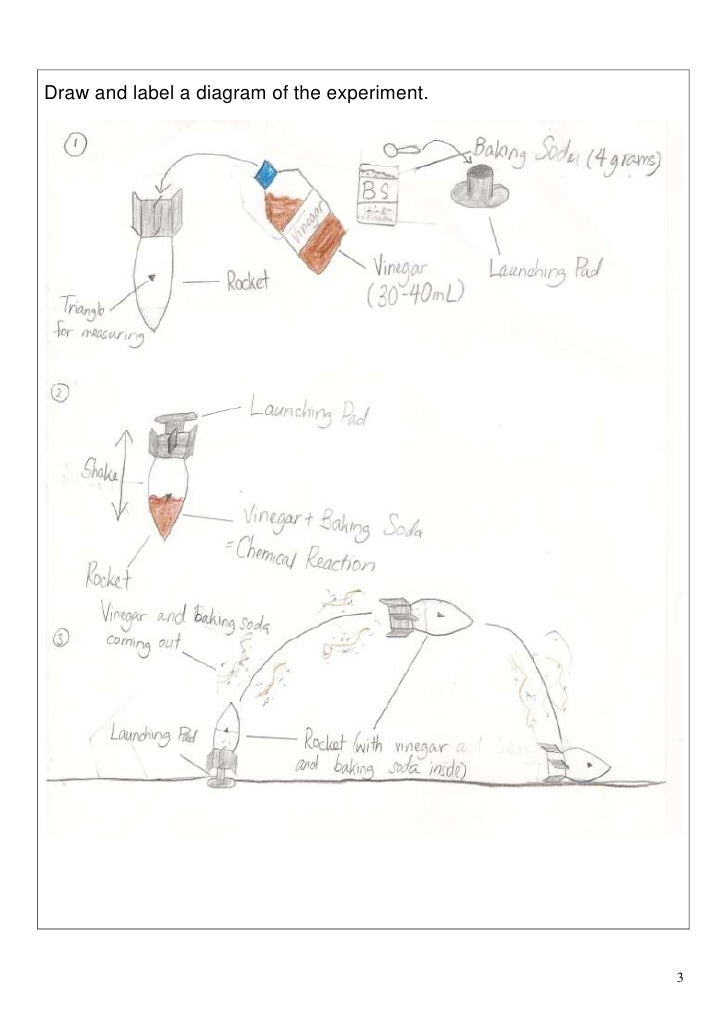



Baking Soda And Vinegar Experiment Report




Baking Soda And Vinegar Powered Car Stephanie Barnes



Blowing Up Balloons With Co2 A Unique Hands On Science Night




How To Inflate A Balloon With Baking Soda Vinegar Youtube




Baking Soda And Vinegar Experiment Report




Baking Soda And Vinegar Balloon Science Project Education Com



Baking Soda And Vinegar Lab Report Buy Essay Online Dissertationgratuite Web Fc2 Com




Baking Soda Vinegar Lab Lesson Plans Worksheets



Www State Nj Us Education 21cclc Casp Lsc Unit1 All Pdf




Inflate A Balloon Career Girls




How To Fill A Balloon With Baking Soda Vinegar 5 Steps With Pictures Instructables



Extension Oregonstate Edu Sites Default Files Documents Lesson1 Overheads Pdf




My Little Corner Of Heaven Pawlak Style Science Fair Project



Blowing Up Balloons With Co2 A Unique Hands On Science Night




Baking Soda And Vinegar Balloons




Balloon Blow Up Science Experiment Children S Museum Of Sonoma County




Balloon Baking Soda Vinegar Experiment For Kids Bilingual Education Activities




Vinegar And Baking Soda Balloon Experiment Happy Brown House




Controlling The Amount Of Products In A Chemical Reaction Chapter 6 Chemical Change Middle School Chemistry




Lesson 27 The Saints Are Expelled From Jackson County Teaching Stripling Warriors




Ol Conservation Of Mass Lab




Middle School Science Expo This Week Wdc Public Schools




Classroom Resources Inflating A Balloon With Chemistry ct




Balloon Blow Up Science Experiment




Is Baking Soda A Compound



Balloon Experiments A Unique Hands On Science Night




Expanding Balloon Room Ppt Download




19 Unit 2 Matter Test Interactive Worksheet By Philippe Menjoulet Wizer Me




Baking Soda And Vinegar Powered Car




Baking Soda Chemical Reactions By Johquaira Rountree




R Chemistryadventure The Textbook By Chemistryadventure Issuu




Self Inflating Balloon Science Experiment




Balloon Baking Soda Vinegar Science Experiment For Kids




Self Inflating Balloon Baking Soda And Vinegar Balloon Experiment Teach Beside Me




Baking Soda Vinegar Lab Lesson Plans Worksheets




Baking Soda And Vinegar Balloon Experiment Science Project Education Com




Korean Cpm English Homework Helper Wrviceonlineessay Web Fc2 Com




Little Scientists



3



I M Posting This Question 4rth Time And Answer Was Chegg Com




Baking Soda And Vinegar Experiment Report




Baking Soda And Vinegar Balloon Science Project Hypothesis



Balloon Experiments A Unique Hands On Science Night




Vaiala Beach School Samoa Introducing Scientist Isabella




Baking Soda Vinegar Balloon Experiment Youtube




Baking Soda And Vinegar S Reaction Perkins Elearning




Westvale Ps Library Blow Up A Balloon Without Using My Lungs I Used Vinegar In A Bottle Amp Baking Soda In The Inside Of A Balloon Amp Attached The Balloon




Ppt Baking Soda And Vinegar Powered Car Powerpoint Presentation Free Download Id




Inflate A Balloon Career Girls




How To Inflate A Balloon Using Baking Soda And Vinegar




Baking Soda And Vinegar Balloons




Data Results Self Inflating Balloons




Title Of Your Project Your Name Purpose Why Are You Conducting The Experiment Or What Question Are You Trying To Answer Ppt Download




Little Scientists




How To Inflate A Balloon Using Baking Soda And Vinegar




Baking Soda Vinegar Balloon Experiment The Go To List




Balloon Baking Soda Vinegar Science Experiment For Kids




Baking Soda Vinegar Balloon Worksheets Teaching Resources Tpt




Baking Soda And Vinegar Balloon Experiment




Science Experiment Making Balloons Expanding Without Blowing Steemit



0 件のコメント:
コメントを投稿